Background
Obinutuzumab is a first-in-class glycoengineered humanized type II anti-CD20 antibody. Immunochemotherapy based on obinutuzumab has demonstrated an advantage in prolonging progression-free survival (PFS) in pts with follicular lymphoma(FL) in a number of clinical trials(Marcus R, et al. N Engl J Med. 2017; Cheson BD, et al. J Clin Oncol. 2018). In Jun, 2021, obinutuzumab came into the market of China. However, few studies have investigated the efficacy and safety of obinutuzumab-based immunochemotherapy aiming at Chinese population. Therefore, we retrospectively analyzed the data of Chinese pts with treatment-naïve(TN) or relapsed/refractory(R/R) follicular lymphoma who received obinutuzumab-based immunochemotherapy in our center, hoping to provide treatment reference for Chinese pts.
Methods
Eligible pts were age ≥18 years with histologically documented, CD20-positive follicular lymphoma (grade 1 to 3a, stage III/IV or stage II with tumor ≥7 cm in the greatest dimension), and at least 2 cycles of obinutuzumab-based treatment. Efficacy was assessed by objective response rate (ORR) and best of response (BOR), with toxicity evaluated based on the Common Terminology Criteria for Adverse Events (CTCAE) (The 5th Edition). In addition, we recorded the incidence and severity of corona virus disease 2019 (COVID-19) infection according to Scheme for Diagnosis and Treatment of COVID-19 (The 10th Trial Edition).
Results
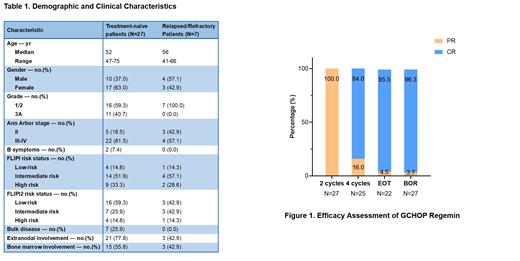
Between Nov 1, 2021 and Apr 1, 2023, the data of 27 pts with TN FL who received G-CHOP (obinutuzumab, cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen and 7 R/R pts treated with GB (Obinutuzumab, bendamustine) regimen was collected. The demographic and clinical characteristics at baseline were shown in Table 1. The median age of TN pts was 52 years, and 63.0% were female. The efficacy evaluation of TN pts was displayed in Figure 1. All of 27 pts achieved partial response (PR) after 2 cycles of GCHOP regimen. Eventually, 21 patiens completed 6 cycles of treatment, with 95.5% (21/22) assessed as complete response (CR) and 4.5% (1/21) maintaining PR. Three pts discontinued treatment due to COVID-19 infection and 2 for severe infections of other reasons. The end of treatment (EOT) ORR was 100% and the BOR of 96.3% (26/27) pts was assessed as CR.
In 7 R/R pts, 5, 1 and 1 pts received 1, 2 and 3 lines of previous treatment regimens, respectively. The median age of this subgroup was 56 years. Totally, 6 pts achieved CR with an ORR of 85.7%, except for one pt who was evaluated as SD after 2 cycles of GB regimen and switched to another regimen. Two pts continued with maintenance therapy of obinutuzumab, and the rest 5 pts went on with autologous hematopoietic stem cell transplantation. By the end of follow-up, no pts showed progressive disease (PD) except for one who died of COVID-19 infection.
All these pts in this study were infected with COVID-19 during the pandemic in China. For TN pts, mild, ordinary, severe and critical cases accounted for 14.8%, 44.4%, 29.6% and 11.1% respectively, and no pt died of COVID-19 infection. The median duration of COVID-19 infection was 23 (14-68) days, and over half of them were tested positive for more than 3 weeks. Of the R/R pts, 57.1% (4/7) presented severe, 14.3% (1/7) was critical and finally died of respiratory failure. The median duration of positive was 26 (21-95) days.
Among pts treated wih GCHOP regimen, common grade 3-5 adverse events included infection (excluding COVID-19 infection) (25.9%) and hematological adverse events consisting of neutropenia (51.8%), thrombocytopenia (22.2%). Grade 3 infection, neutropenia and thrombocytopenia were observed in 14.3% (1/7) R/R pt each.
The median follow-up time of TN pts and R/R pts was 8.9 (12.1-13.7) months and 11.7 (11.2-14.0) months, respectively. The median PFS of both was not achieved, 1-year PFS rate was 100% and 85.7% in TN and RR pts, respectively.
Conclusions
This real-world analysis with small sample size indicates that similar efficacy and toxicity was observed in Chinese patients with FL who were treated with obinutuzumab-based immunochemotherapy to that reported in literature. However, it is noteworthy that long-lasting immunosuppression may result in higher incidence and severity of COVID-19 infection in these patients compared to the general population.
Disclosures
No relevant conflicts of interest to declare.